 The Swimming Pool
Operators
The Swimming Pool
Operators
 The Swimming Pool
Operators
The Swimming Pool
Operators
Resource Page 5 - The Bartier Disinfection Index
- CLICK HERE to go to the Main Welcome Page
- CLICK HERE to go to the Resources Index Page
- CLICK HERE to go to Resources Page 1 - Installation Standards & Recomendations
- CLICK HERE to go to Resources Page 2 - Chemical Safety
- CLICK HERE to go to Resources Page 3 - Water Testing & Analysis
- CLICK HERE to go to Resources Page 4 - Basic Water Chemistry
- You are already at Resources Page 5 - The Bartier Disinfection Index
- CLICK HERE to go to the Resources Page 6 - Common Pool Problems
- CLICK HERE to go to the Resources Page 7 - Frequently Asked Questions
DISCLAIMER:- All the information and advice on these web pages is given in good faith and believed correct and accurate, but we cannot be responsible for any form of loss, damage or injury, however caused, through the use of any information or advice detailed here.
COPYRIGHT:- All the information on these pages are for your PERSONAL USE. You may freely download, and retain anything that appears on this site provided you also retain and include a reference to this site and this copyright notice.. However, any part or whole of any article, text, or information that originates from this site may not be included in any publication, commercial literature, etc., etc., on any other web site, - or in any area where commercial or personal gain is concieved, without the express permission in writing from Malcolm R Bartier.
The Bartier Disinfection Index may be included in any commercial publication provided that (a) It is reproduced in its entirety, complete with reference and credit to its originator - Malcolm Bartier, and (b) that no charge or cost (other than normal printing and distribution costs) are imposed, and (c) that the name of the index remains as the "Bartier Disinfection Index".
Testing for the presence of chlorine will usually result in (a) Recording the FREE CHLORINE, and (b) Recording the TOTAL CHLORINE.
Subtracting the value of FREE CHLORINE from the value of TOTAL CHLORINE will provide a value for COMBINED CHLORINE.
FREE CHLORINE, sometimes refered to as Free Residual Chlorine is, as the name suggests, - a residual of Chlorine available in the pool water for disinfection and oxidation purposes.
COMBINED CHLORINE, sometimes refered to as Chloramines, is the result of Free Chlorine combining with organic debris. Chloramines have very poor disinfection and oxidation properties.
TOTAL CHLORINE is the sum of Free Chlorine plus Combined Chlorine (Chloramines)
It can easily be seen that the most important Chlorine test is for FREE CHLORINE
It is often not appreciated that the disinfection power of a given level of Free Chlorine is dependent on the pH of the pool or spa water. Pool operators frequently wonder why their pool water quality is poor despite having a relatively high level of Free Chlorine present. Very often the poor quality is due to there being a high pH level which reduces or prevents the Free Chlorine from actively disinfecting the water, or the level of Cyanuric acid present has not been taken into account.
When a chlorine doner(other than chlorine gas) is introduced into water, it dissociates into 2 separate parts. Hypochlorous Acid - HOCL, and Hypochlorite Ion - OCL. The HOCL is usuallyable to destroy organisms in less than 2 second, often in less than half a second. The OCL can take up to 25 - 30 minutes to destroy the same types of organisms, and has little or no use in any but very lightly used private pools.
The ratio of HOCL to OCL is dependent on the pH value of the water. The lower the pH, - the more HOCL is available.
At a high pH little or no HOCL is available, and therefore satisfactory oxidation and disinfection take considerably longer, if ever, to achieve.
Unfortunately, the usual test for Free Chlorine records both HOCL and OCL components as Free Chlorine, so unless the pH value is also know it is impossible to tell the percentage of HOCL present in the chlorinated pool water,
- At Ph Values much below 2 pH the percentage of Hydrochloric (Muriatic) Acid is quite high
- At a pH value of 2 pH, the Free Chlorine exists as 75% Hypochlorous Acid (HOCL)and 25% Hydrochloric (Muriatic) Acid (HCL)
- At a pH value of 3 pH, the Free Chlorine exists as 95% Hypochlorous Acid (HOCL)and 5% Hydrochloric (Muriatic) Acid (HCL)
- At a pH value of 4 pH The Free Chlorine consists of 100% HOCL
- At a pH value of 5 pH, the Free Chlorine exists as 99% Hypochlorous Acid (HOCL)and 1% Hypochlorite Ion (OCL)
- At a pH value of 6 pH, the Free Chlorine exists as 96% Hypochlorous Acid (HOCL)and 4% Hypochlorite Ion (OCL)
- At a pH value of 7 pH, the Free Chlorine exists as75% Hypochlorous Acid (HOCL)and 25% Hypochlorite Ion (OCL)
- At a pH value of 7.4 pH, the Free Chlorine exists as 52% Hypochlorous Acid (HOCL)and 48% Hypochlorite Ion (OCL)
- At a pH value of 7.5 pH, the Free Chlorine exists as 48% Hypochlorous Acid (HOCL)and 52% Hypochlorite Ion (OCL)
- At a pH value of 8 pH, the Free Chlorine exists as 22% Hypochlorous Acid (HOCL)and 78% Hypochlorite Ion (OCL)
- At a pH value of 9 pH, the Free Chlorine exists as 7% Hypochlorous Acid (HOCL)and 93% Hypochlorite Ion (OCL)
- At a pH value of 10 pH, the Free Chlorine exists as 3% Hypochlorous Acid (HOCL)and 97% Hypochlorite Ion (OCL)
- At a pH value of 11 pH, the Free Chlorine exists as 0.03% Hypochlorous Acid (HOCL)and 99.97% Hypochlorite Ion (OCL)
At pH Values above 11 pH the Free Chlorine exists almost entirely of OCL - the Hypochlorite Ion, and although a test kit can indicate a substantial level of Free Chlorine available,it can be see that at high pH values the free chlorine will have very little or no oxidation and disinfection properties.
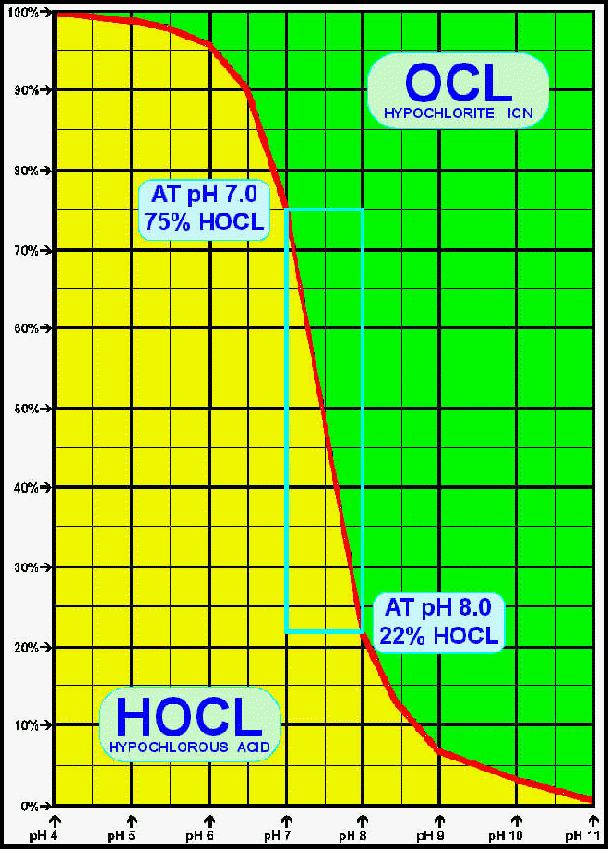
In the above graph, the horizontal line represents pH values from 4.0 pH (left) to 11.0 pH (right). The vertical line runs from 0% (base) to 100% (top). The blue rectangle shows the range 7.0 pH to 8.0 pH and indicates how at 7.0 pH there is 75% available HOCL - which falls to 22% at 8.0 pH. This graph shows how important it is to maintain the correct pH value - usually 7.4pH since this is the pH of the human eyes.
Cyanuric acid (chlorine Stabilizer) in chlorinated water, whether introduced separately, or present through the use of stabilized chlorine (dichlor granules or trichlor tablets), will reduce the amount of available HOCL. At low levels of Cyanuric Acid there is very little effect, but as the cyanuric acid level increases, the disinfecting and oxidising properties of the available chlorine will become progressively reduced. High levels of cyanuric acid cause a situation known as "Chlorine Lock" - when even very high levels of chlorine become totally locked up with the stabilizer and are rendered usless.
In order to make the assesment of the oxidising and disinfecting properties of chlorinated water a simple matter, we have developed and produced THE BARTIER DISINFECTION INDEX.
First the water is tested for Free Chlorine and pH, and a note made of the results. The pH reading is compared to a chart to see the pH FACTOR. This pH Factor is then multiplied by the actual Free Chlorine reading to give the Index Value. If Cyanuric acid is present, the level of CYA is deducted from the Index Value. The final result is compared to a chart again, to indicate the level of Oxidation and Disinfection available.
The Bartier Disinfection Index has been in use since 1994, and has proved invaluable to an increasing number of pool operators.
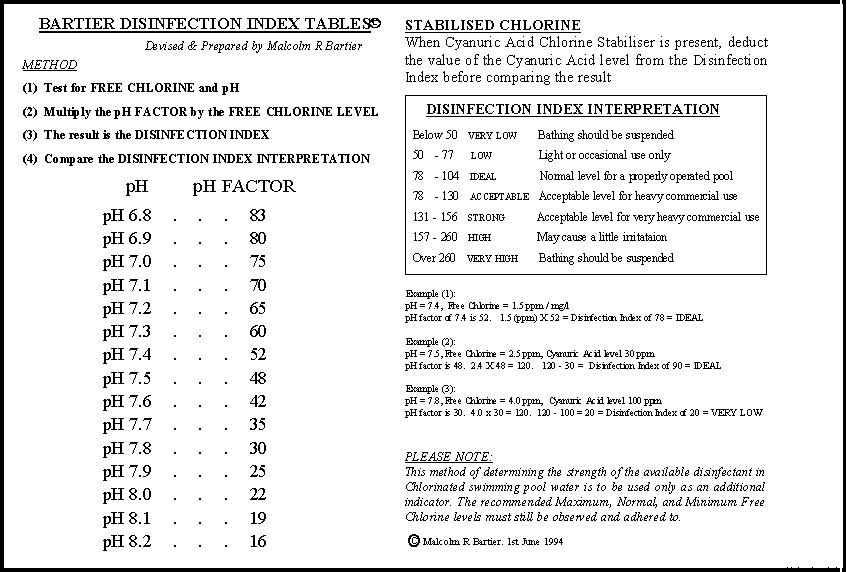
The above is a copy of the Bartier Disinfection Index, and has proved invaluable troubleshooting problem pools. It is also extremely useful as an aid to explaining the importance of pH control
I should be remembered that a well run large public swimming pool may well be able to operate safely with Free Chlorine levels of 1 ppm, or even slightly less. This Index was developed as an aid to CORRECTING pool disinfection problems, particularly hotel and school pools, and often those with unreliable or even non existant automatic chemical dosing equipment.
This Index is most suitable for hotel and school pools, and larger pools with a history of poor disinfection.
- CLICK HERE to go to the Main Welcome Page
- CLICK HERE to go to the Resources Index Page
- CLICK HERE to go to Resources Page 1 - Installation Standards & Recomendations
- CLICK HERE to go to Resources Page 2 - Chemical Safety
- CLICK HERE to go to Resources Page 3 - Water Testing & Analysis
- CLICK HERE to go to Resources Page 4 - Basic Water Chemistry
- You are already at Resources Page 5 - The Bartier Disinfection Index
- CLICK HERE to go to the Resources Page 6 - Common Pool Problems
- CLICK HERE to go to the Resources Page 7 - Frequently Asked Questions
r
END OF RESOURCE PAGE 5